The number of research and development programmes is increasing year by year with a strong focus in three key areas: respiratory, neonatology and special care.
Respiratory is a core area of R&D expertise, which has delivered significant value for the company and continues to expand to meet areas of unmet medical need in asthma, Chronic Obstructive Lung Disease (COPD) and other respiratory diseases
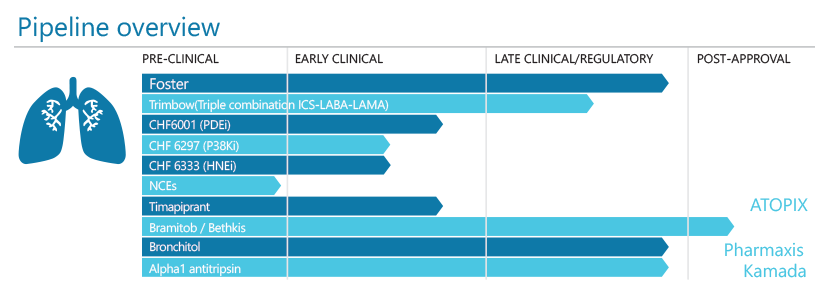
Foster is a key revenue driver for the future of the company, but it also remains a highly significant element of R&D activity as we roll out a powerful life cycle management.
There is a substantial commitment to a very attractive therapeutic opportunity with our Triple programme. Our product candidate contains three active ingredients in a single inhaler that will ensure effective anti-inflammatory therapy combined with maximum bronchodilation throughout the entire respiratory tree at each inhalation.
The exciting pipeline of new molecules continues to show significant promise. These programmes include novel anti-inflammatory and bronchodilator molecules, and also innovative “double” active molecules addressing two different pharmacological targets.
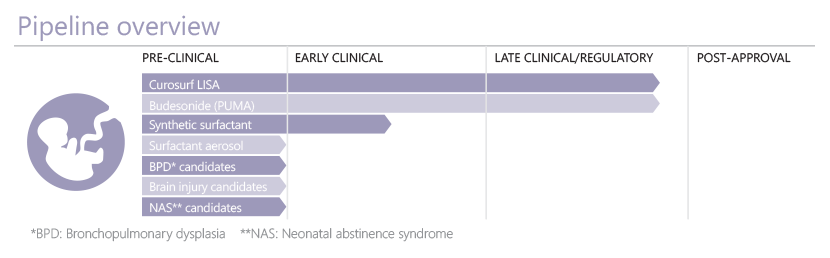
Through the clinical success of Curosurf in treating neonatal respiratory distress syndrome, Chiesi has established an impressive platform in neonatology. In this area of high unmet medical need R&D programmes include novel and less-invasive methods for the targeted delivery of Curosurf to the lung of the infant. The proprietary synthetic surfactant is undergoing clinical trials with emerging data supporting the safety, efficacy and clinical utility of this potential new addition to surfactant replacement therapy options.
As we expand our pipeline behind these ground-breaking respiratory-focused products, new programmes have been developed and added for candidates for the treatment of other neonatal conditions. For example, there are currently no effective approved pharmacological therapeutics for neonatal brain injury: a relatively common condition associated with significant levels of morbidity and mortality in newborns.